Science Plan, Operations and Outcomes
The BLOOFINZ science plan
From the start, BLOOFINZ took an “adaptive” strategy, meaning that there was not a pre-determined design of sampling locations, just a general concept of what would be done at locations determined by environmental conditions at the time. Tuna larvae and environmental conditions would be surveyed with net and CTD sampling. Water parcels with concentrations of tuna larvae would be marked and followed by satellite-tracked surface drifters. Elaborate multi-day process experiments (called “Cycles”) would be done using two additional satellite-tracked drift arrays: 1) to incubate bottles at different depths to measure primary production, nutrient uptake, phytoplankton growth and grazing over the euphotic (lighted) zone down to about 100 m, and 2) to measure the sinking loss of organic matter from the euphotic zone into sediment traps. Additional sampling and shipboard experiments over the 4 days and 4 nights in the drifting water parcel would also be done to close input and output budgets and to parameterize models of food-web flows. Cycle experiments were expected to run on very tight schedules 24 hours per day (see example — Appendix I).
In addition to experimental cycles, BLOOFINZ had a strong component devoted to continuous measurements of the near-surface environment where blue fin tuna larvae reside (upper 25-30 m). Using the ship’s flow-through seawater system (pumped from an inlet at the ship’s bow) or from a towed trace-metal (uncontaminated) sampling system (the TM “fish”) towed at the end of a 19-ft pole, we had a large number of instruments that imaged plankton composition, analyzed pigments and photo-physiology or estimated primary production and nitrogen fixation rates. On long-distance transects to and from the study site, we also towed a CPR (Continuous Plankton Recorder) to measure abundances and community composition of zooplankton. Part of the strategy of the cruise was to use these capabilities to measure changes in the properties of the environment across circulation features to assess differences in the habitat quality for feeding, growth and survival of larval tuna.
Lastly, we did not go into the study region blindly. Barb Muhling of the NOAA lab in Santa Cruz provided daily satellite products of sea-surface height and currents, temperature, chlorophyll and predictive model output of where tuna larvae might be found in the region. These began in January, weeks before we reached the study area, and continued uninterrupted for the study duration. Figure 1 (below) shows the sea-surface height and surface current map as we saw it on 1 Feb., the day before the CTD was lost. We had just spent 4 days on the purple line (each dot is one day) coming westward across the shallow NW Australia coastal margin from Darwin and were now over deep water. We had sampled the southern-flowing water on the map at ~120° and 115°E longitude and found no tuna larvae coming into the region from the Indonesian side. The ship is the red dot, and we are making our way to the site where an historical bluefin larvae study was done 35 years ago in 1987 (the red diamond). Though we are still ~200 miles away from that site at the time, we can see that there is a small counter-clockwise eddy to the west and with a strong eastward flow into the diamond area. That is where we hope to find our first significant catch of larvae and deploy a patch marker to start our first cycle experiment.
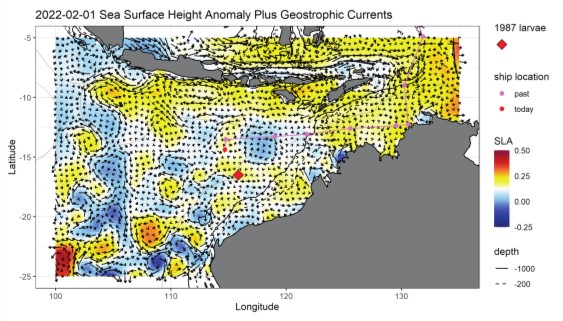
Figure 1. SSH and surface currents, 1 Feb. 2022
Adaptability can mean differ things. We were prepared to respond and adapt to environmental conditions. In this case, however, we also had to deal with a major equipment loss and winch problems.
How the cruise unfolded
Figure 2 is a screen shot of the shipboard navigational chart showing the region divided into Indonesian and Australian EEZs (Exclusive Economic Zones) and International waters and our cruise track through it. We had permits to work in Australian water, but not the Indonesian side. Our original idea was to follow the Australian coastline south after we got off the wide shallow shelf west of Darwin, but a storm in the south kept us going west until ~114°E, where we turned toward the historical larval study site (diamond in Fig. 1). We found abundant but small early-stage larvae there, marked the patch with a drifter, and prepared to start Cycle 1. The CTD was lost on the first cast. C1 was started a day later with Mighty Mini and a revised plan that accounted for reduced water sampling volumes. C1 did, however, run successfully for the full 4 days, and we left the marker drifter in the patch when we departed, so that we could return later to see growth of the young larvae and their survival success.
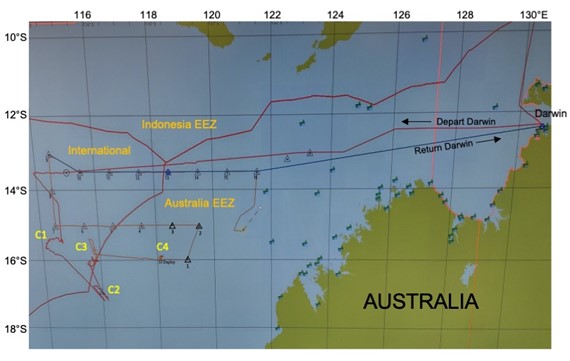
Figure 2. Screen shot of cruise track. C1-C4 are locations of experimental cycles. Triangles are transect sampling stations
Australian coastline south after we got off the wide shallow shelf west of Darwin, but a storm in the south kept us going west until ~114°E, where we turned toward the historical larval study site (diamond in Fig. 1). We found abundant but small early-stage larvae there, marked the patch with a drifter, and prepared to start Cycle 1. The CTD was lost on the first cast. C1 was started a day later with Mighty Mini and a revised plan that accounted for reduced water sampling volumes. C1 did, however, run successfully for the full 4 days, and we left the marker drifter in the patch when we departed, so that we could return later to see growth of the young larvae and their survival success.
After leaving C1, we sampled to the southwest, finding fewer larvae but conducting a successful Cycle 2. Then we returned to the marker drifter left at the end of C1. Figure 3 shows the screen that kept track of drifter positions and trajectories (the googlie eyes are ship art drawn onto a piece of yellow tape and stuck to the screen; written labels for Cycles 1-4 and the ship were added later). The patch markers are the black lines; the sediment trap drifters are blue; and experimental incubation drifters are orange. All of the drifters are clearly in a circulation eddy that is taking them in a large anti-clockwise loop. When we left the patch marker, it was heading west and south, but it boomerangs back to us for Cycle 3 and then turns north. At the end of Cycle 3, we headed east for Cycle 4, which went well until Mini lost her bottle controller. Cycle 4 ends prematurely and our plan to do a final Cycle 5 in a northern area with few larvae cannot be done without a water-collecting CTD.
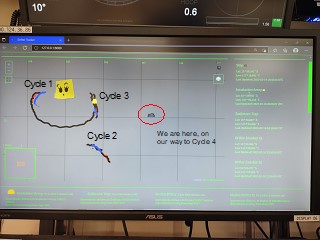
Figure 3. Screen shot of the drifter tracker screen.
When Mini cannot be repaired, a new plan is hatched at a P.I. meeting the following morning – we will spend the remaining week doing transect sampling, first westward along 15°S, then returning eastward along 13.5°S. These are the 16 locations with triangle symbols in Fig. 2. The loss of Cycle 5 is a disappointment, but not a catastrophe. We still are able to sample larvae and zooplankton with nets, do underway sampling with the surface seawater systems, collect smaller amounts of deeper water with hand-attached Go-Flo bottles that Pete has brought for his trace metal work, and do basic CTD profiles with skinny Mini. Near-surface seawater is available in unlimited quantity for experimental incubations. Proceeding westward along 15°S, we are also able to avoid a large tropical storm that is building north of us. We do 2 or 3 stations each day, short ones with only nets and CTD profiles, but a long station each afternoon-to-evening to run through a full suite of sampling and set up experiments. Five deck incubators are soon filled to their brims with experimental bottles. This change in approach has the advantage of providing a broader comparative spatial context to the experimental cycles that we conducted in the south, and might well end up being more helpful for us to understand the system than our originally planned C5.
The story beneath the story
While Figures 1-3 show dots on maps where the BLOOFINZ cruise went, they do not explain why we chose those places over others. For this, we need to look at the depth and shape of the seafloor. Our study area overlies the Argo Abyssal Plain, a deep basin of over 5000 m (more than 3 miles). In research that some of us teamed up to do on Atlantic bluefin tuna in the Gulf of Mexico, we found that patches of larvae could be traced to origins along the steep outer slopes of a similarly shaped deep basin. Here, we use that insight to focus on waters overlying the southern slope region of the Argo basin, where we might reasonably expect adult bluefin tuna that migrate up the western coast of Australia to first enter the spawning region and to aggregate for foraging and reproduction. Consistent with this view, larvae were found in all cycle experiments in this region, the highest density at C4, and on one occasion a large mass of recently spawned eggs was collected (whether SBT or not will be determined by genetic analysis). However, our survey samples also revealed that larvae are broadly spread throughout the region by currents and eddies. In particular, standardized net tows gave substantial catches of larvae along Joey Rise to the north (“joey” being the name for a baby kangaroo, which the shape of the rise suggests, relative to the larger Roo Rise above it) suggesting that adult bluefin spawners also congregate around this feature.
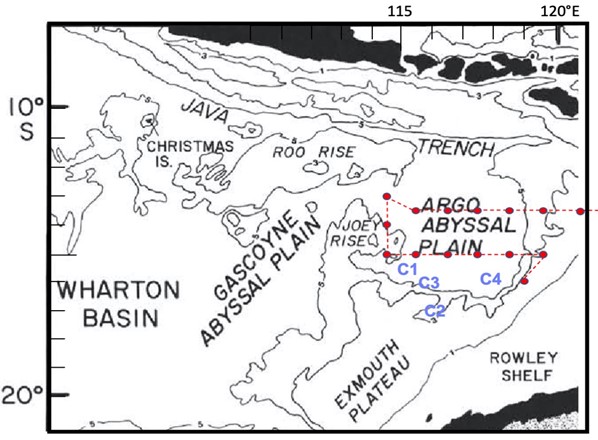
Figure 4. Seafloor bathymetry with Cycle 1-4 locations superimposed
in blue and survey transect stations in red.
The full extent of cruise findings will likely take a few years to analyze and unravel, but we have some early indications that the ITF spawning region is substantially more productive than we thought and relative to the Gulf of Mexico. It also appears that southern bluefin larvae grow fast and robustly at water temperatures (30-31.5°C = 86-89°F) that are well above the thermal optimum (25-26°C = 77-79°F) and possibly even lethal for Atlantic bluefin larvae.
Our good fortune and thanks
Part 2 of this 3-part narrative focuses on cruise challenges that seemed (and were!) more consequential at the time than in the final analysis. That we ended up with a very successful cruise despite the challenges can only be attributed to an abundance of good luck to compensate for the bad, all largely out of our control.
The weather: SBT spawning occurs during NW monsoon and the cyclone season of northern Australia, and we expected to endure a substantial amount of rough weather in our study region. Given our equipment issues, operating the CTD from the stern of the ship could have been especially difficult or dangerous under high seas. However, other than the two storms at the beginning and end of the cruise that we were in position to easily avoid, we had calm seas and beautiful working conditions throughout. No time was lost to weather, and we never got close to a discussion about shutting down operations. Thank you!
The COVID reprieve: Two of the areas that functioned flawlessly and contributed greatly to the success of the cruise were larval tuna sampling (Team Tuna) and flow-through instrument measurements. It is difficult to imagine how the cruise would have gone, certainly much less successful, without the energy and expertise of Raul, Jose, Joaquim and Alejandro. We are extremely grateful to SIO ship operations and to NSF for agreeing to push back the schedule, at substantial cost to them, to allow these folks to pass the official recovery period and join the cruise. It was a key decision done behind the scene, and the answer could easily have been “no”. Thank you!
The fickle finger of funding: While the core BLOOFINZ program was funded as one multi-institutional collaborative research project, Pete Morton’s trace metal (TM) component was a late addition that went through a separate NSF review. That it was funded was a great stroke of good fortune. It provided vital infrastructure (TM sampling pole and fish) to support underway sampling and shipboard iron-limitation experiments. When the CTDs and other winches failed, the small Hawboldt winch and Go-Flo bottles that were brought for Pete’s work became the fallback options that kept net tow and subsurface water sampling going. Thank you!
We also have some special thanks for those whose efforts supported BLOOFINZ science.
Res Techs: The flip side of “good” weather is that it can be hellishly hot and humid on the iron deck of a ship during summertime in the tropics. The science party could come and go as their turns came up in the activity schedule, but Jeremiah and Caitlyn were on the deck for their 12-h shifts all day, every day – not only getting by, but having to be fully attentive to safety issues, bridge-lab-winch communications and directing each operation, as well as diagnosing, repairing and re-terminating equipment when there were failures. Toughest jobs on the ship (or at least rivaling that of head cook Richard’s). We owe them a huge debt of gratitude for their competence, endurance and getting us through. Thank you!
Captain and crew of R/V Roger Revelle: This was Captain Eric’s first command of a cruise with an international port call, his first experience with some of the unusual over-the-side operations of our program (CPR, daily drifter recoveries/deployments) and he was called into service on a least a couple of occasions to administer first aid and medical judgement. He handled all of these roles with skill and grace, with a warm and welcoming presence and an interesting story for every occasion. That attitude was also reflected in his crew. We greatly appreciate the efforts of all who facilitated the science directly, the ship maintenance on which the science depends, and those, in particular, (science party and crew; Zack for midnight snacks) who bolstered Richard’s heroic efforts by helping to fill gaps in cooking and cleanup. To maintain a COVID-free bubble, all were burdened by masking up for almost a full month. The difference is that we temporary visitors will be leaving Revelle in a few days, while the crew will be putting them back on as they welcome a new group aboard. Thank you!
Mike Landry


